MESA Denitrification Project
April 2019 to June 2021
GCCC Principal Investigator: Katherine Romanak
Introduction
GCCC’s work for the MESA project focused on creating biologically-active laboratory microcosms that could achieve denitrification. Temporal variations in soil gas fluxes of N2, O2 and CO2 and their isotopic compositions could then be conducted with a focus on the transition from simple aerobic respiration to the anoxic process of denitrification and through to methanogenesis. The information gained can be applied to understanding, measuring and quantifying complex vadose zone processes.
We utilize and are developing a “process-based” soil gas ratio approach to illuminate processes occurring in the vadose zone [1-3]. Processes are identified based on soil gas ratios that represent the stoichiometry of each process. Stoichiometric approaches are common in the assessment of reactions in aqueous geochemistry but are not commonly used for soil gas analysis. However, we have found that such an approach can be extremely powerful for unraveling the geochemical complexity of the vadose zone, and when coupled with isotopic measurements could provide a useful paradigm in vadose zone science.
The current process-based assessment matrix includes the aerobic processes of respiration, gas dissolution and methane oxidation using the ratios of O2 versus CO2, CO2 versus N2, and CO2 versus N2/O2 however they have not, until this point included the important anoxic processes of denitrification or methanogenesis (specifically through acetate fermentation, or CO2 reduction) common to waterlogged systems. Thus, our initial work focused on developing laboratory capabilities to investigate the transition from aerobic biological processes of respiration to the anoxic processes of denitrification and methonogenesis with the understanding that once these processes were achieved, the geochemical and isotopic variations could be fully characterized and investigated.
Background on a Process-Based Approach
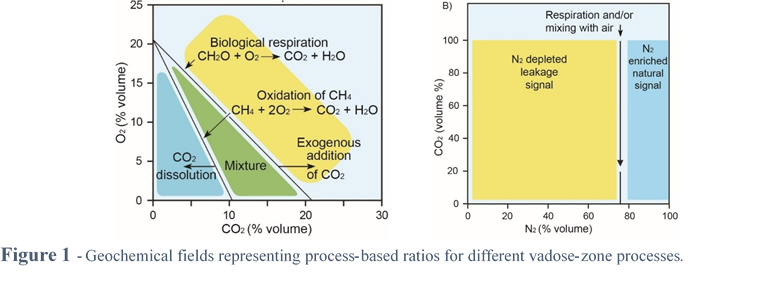
Beginning with the composition of air, processes in the vadose zone will alter the geochemistry of soil gas in predictable ways that can be used to identify the processes using examination of several different relationships in sequence; O2 versus CO2, CO2 versus N2, and CO2 versus N2/O2 (Figure 1).
Biologic respiration utilizes O2 as the terminal electron acceptor for energy production and produces CO2 as a by-product according to the reaction:
CH2O + O2 → CO2 + H2O
When the supply of organic matter outpaces that of O2, anaerobic bacteria utilize alternate electron acceptors (SO42–, NO3–, Fe3+ and oxyhydroxides) when available. In the absence of these electron acceptors, CH4 is produced. When CH4 migrates into oxic zones, or environmental change results in an influx of O2 into a previously anoxic, CH4-producing environment, CH4 is oxidized to CO2 according to the following equation:
CH4 + 2O2 → CO2 + 2H2O
These common biologic processes result in deviations from atmospheric concentrations for CO2 and O2 along a trend with a slope of -1 for biologic respiration and -2 for methane oxidation (Figure 1 left). Addition of CO2 such as might migrate from depth in a volcanic area will create CO2 concentrations larger than would be expected from corresponding O2 concentrations based on these relationships. Alternatively, CO2 concentrations less than those predicted from O2 concentrations may signal a loss of CO2 due to dissolution [4, 5].
Because gas concentrations are measured in percent (by volume or molar), any non-reactive addition or subtraction of a gas component will, by definition, dilute or concentrate (respectively) all other gas components in a gas mixture (Figure 1 right). N2, a relatively non-reactive but major component in air and soil gas can be used to indicate this process. Used in conjunction with the relationships between CO2 and O2 described above, CO2 that shows a negative correlation with N2 signals dilution by input of exogenous gas [6, 7] and CO2 that shows a positive correlation with N2 may indicate dissolution of CO2 [5].
Denitrification is a type of anaerobic respiration that uses nitrate as an electron acceptor and is a microbially-facilitated process involving the stepwise reduction of nitrate (NO3-) to nitrite (NO2), nitric oxide (NO), nitrous oxide (N2O), and, eventually, to dinitrogen (N2) [8] The complete denitrification process can be expressed as a redox reaction:
2 NO3− + 10 e−+ 12 H+ → N2 + 6 H2O
Denitrification usually occurs when oxygen supply is limited in soil which is wet or waterlogged and depends various controlling factors: presence of nitrate, temperature, soil wetness, and dissolved carbon. Denitrification only occurs when nitrate is present. Wet soils are generally the trigger for denitrification to occur. Denitrification can proceed in aerobic conditions, but to a relatively insignificant degree. The end product of denitrification also depends on supply of soluble organic carbon. With adequate supply of soluble organic carbon, N2 gases are produced. Otherwise, other intermediate products, such as N2O and NO may be released. For this portion of Task 2, we focus N2 gas as the end product of denitrification.
When nitrogen is exhausted and under anaerobic conditions methanogenesis will proceed either by acetate fermentation or by CO2 reduction. [9]. The overall stoichiometry of these reactions is
2CH2O → CH4 + CO2
where CH4 and CO2 are produced in a 1:1 ratio in the absence of oxygen. Whereas these two types of methanogenesis will result in different isotopic signature, for the preliminary purposes of this task, we consider only the ratio of CO2 to CH4.
Results and Conclusions
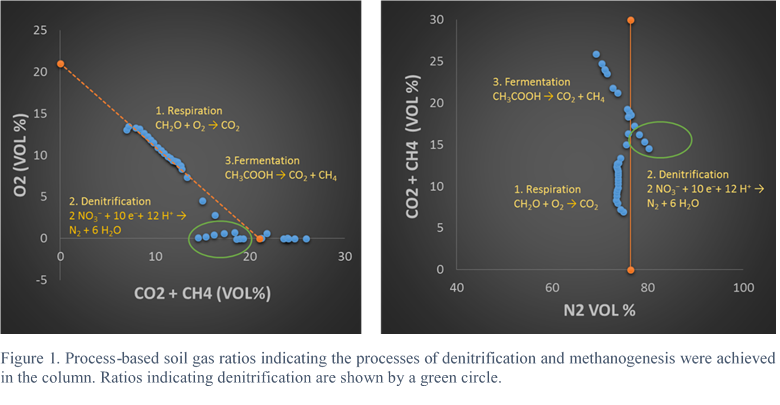
Results are shown in Figure 3 and Table 2. Table 2 shows soil gas concentrations during three phases 1) open system without heat, 2) closed system without heart and 3) closed system with heat applied. During open system conditions, O2 decreased from 13.04 to 7.33 vol % while CO2 increased from 6.97 to 13.38 % indicating aerobic respiration. N2 remained virtually unchanged ranging within the precision of GC measurement between 74.89 and 73.43%. During this phase no methane was detected.
Upon closing the system, O2 decreased from 4.5 to 0 % signaling anaerobic conditions. CO2 generally continued to rise from 15.01 to 19.29 % with a momentary decrease to 14.53 %. Trace amounts of methane became detectable ranging between 0.0007 and 0.002% (7 to 20 ppm). N2 increased from 75.57 to 80.25% signaling input of N2 through denitrification. Upon applying heat to the closed system, CO2 again rose sharply from 15.43 to 35.0% while methane also rose from 0.0012 to 0.6408% (12 to 6408 ppm). N2 decreased from 79.36 to 35.66 indicating dilution from the addition of gas components through the breakdown of organic matter through fermentation. Thus, it appears that both denitrification and methanogenesis were achieved in the column and were clearly indicated by process-based gas ratios. The next steps will be to upscale the experiment and further verify and investigate the processes with isotopic analysis.
References
1. Romanak, K.D., et al., Process‐based approach to CO2 leakage detection by vadose zone gas monitoring at geologic CO2 storage sites. Geophysical Research Letters, 2012. 39(15).
2. Romanak, K., et al., Assessment of Alleged CO2 Leakage at the Kerr Farm using a Simple Process-based Soil Gas Technique: Implications for Carbon Capture, Utilization, and Storage (CCUS) Monitoring. Energy Procedia, 2013. 37(0): p. 4242-4248.
3. Dixon, T. and K.D. Romanak, Improving monitoring protocols for CO2 geological storage with technical advances in CO2 attribution monitoring. International Journal of Greenhouse Gas Control, 2015. 41: p. 29-40.
4. Romanak, K.D., Vadose-Zone Geochemistry of Playa Wetlands, High Plains, Texas. 1997, University of Texas, Austin.
5. Romanak, K.D., et al., Process-based approach to CO2 leakage detection by vadose zone gas monitoring at geologic CO2 storage sites. Geophysical Research Letters, 2012. 39(15): p. L15405.
6. Romanak, K., et al., Process-based soil gas leakage assessment at the Kerr Farm: comparison of results to leakage proxies at ZERT and Mt. Etna (in Press). International Journal of Greenhouse Gas Control, 2014.
7. Riding, J. and C. Rochelle, Subsurface characterization and geological monitoring of the CO2 injection operation at Weyburn, Saskatchewan, Canada. Geological Society Special Publication, 2010. 313(1): p. 227-256.
8. Wolf, I. and R. Brumme, Dinitrogen and Nitrous Oxide Formation in Beech Forest Floor and Mineral Soils. Soil Sci Soc Am J, 2003. 67(6): p. 1862-1868.
9. Chanton, J., et al., Carbon and hydrogen isotopic effects in microbial methane from terrestrial environments. Stable isotopes and biosphere-atmosphere interactions, physiological ecology series, 2004: p. 85-105.
Last Updated: June 30, 2021



