2018–2022 Sandtank Lab Background
The GCCC’s former Ph.D. student, Prasanna Krishnamurthy, spearheaded a novel approach to visually and numerically analyze fluid flow. By programming the way that glass beads (sand) are fed into a glass chamber, sedimentary structures (e.g., crossbedding and ripple-like deposits) are manufactured under high precision to become subsurface analogues.
Why is this important?
Today, we observe sediment moving across Earth’s surface and know that sediments organize in naturally occurring patterns (e.g., sedimentary structures) due to the action of wind and water. We know that the intensity of sediment transport, fluid conditions, etc. form specific sedimentary structures in natural environments and that these sedimentary structures are often preserved in layers of sediment. When layers of sediment become buried, compacted, and cemented they undergo rock-forming processes (e.g., lithification) over time to form what geologists refer to as sedimentary rock. Sedimentary rocks are naturally great reservoirs, since they are often porous and permeable. In the subsurface, reservoir rocks are critical for storing drinkable water, oil, and gas, etc., but they are also key for storing supercritical CO2. Experimenting with fabricated sand packs that mimic natural sedimentary features, found within reservoir rocks, allow GCCC researchers to observe and measure how supercritical fluids react to variations of porosity, permeability, and bedding patterns that are analogous to how CO2 moves in subsurface reservoirs.
In geologic carbon storage injection sites, when CO2 is injected, the pressurized supercritical fluid travels from the injection site to pore spaces in the surrounding reservoir rocks until a variety of forces or a natural sealing layer, or cap rock, stops its flow.
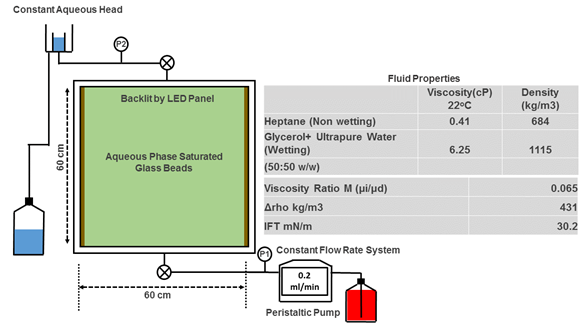
In the experimental setup, fluid migration experiments were conducted using heptane and glycerol + ultrapure water, which mimic many of the attributes (like density and viscosity contrasts and interfacial tension) of supercritical CO2 and brine water in a real storage reservoir. At room temperature, humidity, and pressure, the experiment starts with the CO2 analogue, heptane, being injected into the bottom of the glass chamber by a pump.
Low flow rates ensure that the fluid is driven mainly by the upward force of buoyancy. The fluid travels upwards through the pores in between the beads until it reaches the bottom of the next layer of beads, continuing with some lateral migration, trapping and backfilling, until the fluid reaches the top of the chamber.
Back lighting and image processing are used to visually capture the process as well as calibrate and quantify the trapped fluid saturation during the experiment.

Flow imaging takes places in a dark room setup including a monochrome CCD sensor camera and the flow well backlit by a LED panel.
By observing the behavior of a CO2 analogue in the lab, scientists are able to predict the behavior of supercritical CO2 according to different attributes of reservoir rocks before any is injected underground—whether and how far it will move laterally and vertically—is helpful in understanding what sedimentary features within a potential reservoir will maximize the amount and success of CO2 storage.
Read "Mimicking Geologic Depositional Fabrics for Multiphase Flow Experiments" published in AGU's Water Resources Research, outlining some results of this study.
The automated packing system
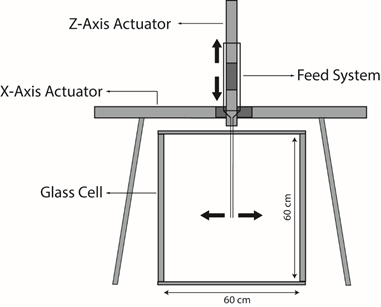
The experimental setup
Last updated: January 23, 2026



